From CRS Today
Divided FDA Advisory Panel Votes that Benefits of Kamra Inlay Outweigh Risks
The FDA’s Ophthalmic Devices Panel voted on June 6 that the benefits of the Kamra inlay (AcuFocus) outweigh the risks for patients with vision loss as a result of presbyopia. The voting was divided, however, and concerns about safety were raised.
The FDA panel reviewed clinical data from 508 patients implanted monocularly with the inlay in the US IDE clinical trial. Of the eight panelists, seven voted that the implant is effective in patients who meet the criteria specified in the proposed indication. On the question of the implant’s safety in patients who meet the criteria specified in the proposed indication, there was a 4-4 tie. Panel chairman Neil M. Bressler, MD, cast a tiebreaker vote by voting “no. ” The chairman only votes in case of a tie. On the question of whether the benefits outweigh the risks, four panelists voted “yes,” three voted “no,” and one abstained.
John A. Vukich, MD, a partner at the Davis Duehr Dean Center for Refractive Surgery in Madison, Wisconsin, and a clinical investigator for AcuFocus, presented efficacy data from the 36-month prospective, open-label, multicenter pivotal trial. The patients included in the study all had uncorrected near visual acuity worse than 20/40 but better than 20/100 and a best-corrected distance visual acuity of 20/20 or better. The primary effectiveness endpoint—75% of the subjects achieving uncorrected near visual acuity of 20/40 or better at 12 months—was met (83.5%). Participants had a mean 2.9-line gain at month 12, Dr. Vukich said.
“In the clinical trial, the average loss of uncorrected distance vision was only 3 letters,” Dr. Vukich said in an interview with Cataract & Refractive Surgery Today. “So we give up a little bit, but that’s uncorrected vision. The average loss of BCVA was only 1 letter, and so we’re giving up very, very little, and in return, we’re maintaining excellent binocularity at distance, excellent stereopsis, and excellent binoncular submmation because both eyes are still functioning at distance together as a team. And we’re not penalizing the implant eye for the distance-related activities. And yet (the implant) still provides the near acuity that allows people to read and do things that they want up close.”
The FDA will consider the advisory panel outcome as part of its own determination of the benefit-risk of AcuFocus’ premarket approval submission for the Kamra inlay, according to a company news release.
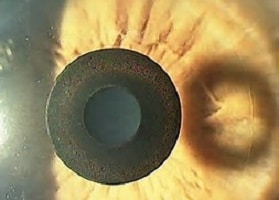
The Kamra inlay is a permanent implant intended to improve the near vision of presbyopic patients. The inlay is implanted into the patient’s nondominant eye and uses the principle of small-aperture optics to improve near vision with minimal change in distance vision. The Kamra poses little or no risk to distance visual acuity, is removable, and sustains its effect over time, according to AcuFocus.
The entire proposed indication is “for the improvement of near and intermediate vision in presbyopic patients who require near or intermediate correction. The inlay is intended to be placed intrastromally in the cornea, on the visual axis, by way of a femtosecond laser-created pocket using a spot/line separation of 6×6 µm or less. The inlay should be placed at a depth equal to or greater than 180 μm.”
Kamra has been on the market outside the United States since 2009, with approval in 50 countries, and AcuFocus estimates that nearly 20,000 Kamra inlays have been implanted.
